
The standard entropy of reaction helps determine whether the reaction will take place spontaneously. Solution for From the table of the standard entropy values, calculate the standard entropy changes for the following reactions at 25☌. Similarly, liquids have higher entropies than solids because molecules in a liquid can interact in many different ways. Gases, which serve as efficient vehicles for spreading thermal energy over a large volume of space, have much higher entropies than condensed phases. Standard Entropy: The absolute entropy of a substance at standard state (25C, 1 atm, 1M, etc.) Expressed as J/K. These trends are consistent with the principle that the more disordered a substance, the greater its entropy. For example, sodium, which can be cut with a knife, has almost twice the entropy of iron, which cannot be easily cut. Tables of Chemical Thermodynam ics Properties, Phys.
Standard entropy table free#
There is an inverse correlation between the hardness of a solid and its entropy. Table 8 gathers the calculate d values of the entropy, standard mola r entropies and Gibbs free energy of. Instead of storing entropy, store the mnemonic generated from the entropy. The carbon atoms in diamond are tightly locked in a three-dimensional lattice, preventing them from vibrating around their equilibrium positions. Graphite, which is built up of loosely-bound stacks of hexagonal sheets, soaks up thermal energy twice as well as diamond. Although both diamond and graphite are types of carbon, their entropies differ significantly. The standard molar entropy of NH3 g is 19245 J K1 mol1 at 29 K and its heat capacity value given by eqn 225 with the coefficients given input Table 22 in member Data Section. Standard entropies for electrically neutral substances are defined to be relative to the crystal state at 0°K so S for common hydrogen related species is H 2 130.6 H 114.6 H 2 O 188.7 OH 183.6 H + 0 OH-10.
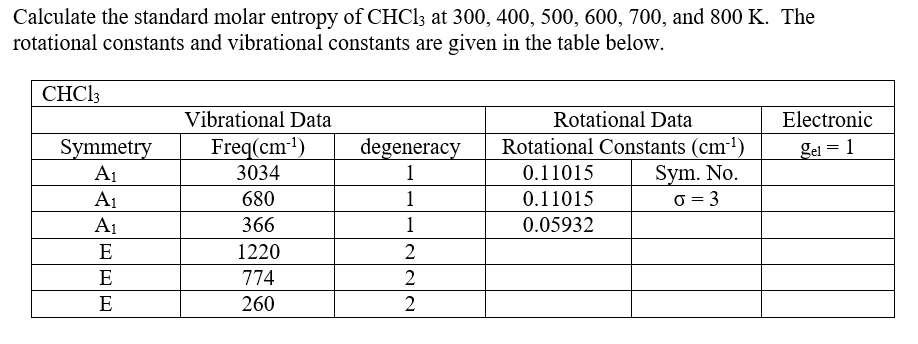
The entropies of solid elements are strongly influenced by the type of atom packing in the solid. Standard entropies for ions are defined by convention as relative the the hydrogen as taken to be zero. The entropies of the diatomic and polyatomic molecules show the additional effects of rotational quantum levels. For the noble gases, this is a direct reflection of the principle that translational quantum states are more closely packed in heavier molecules, allowing them to be occupied.
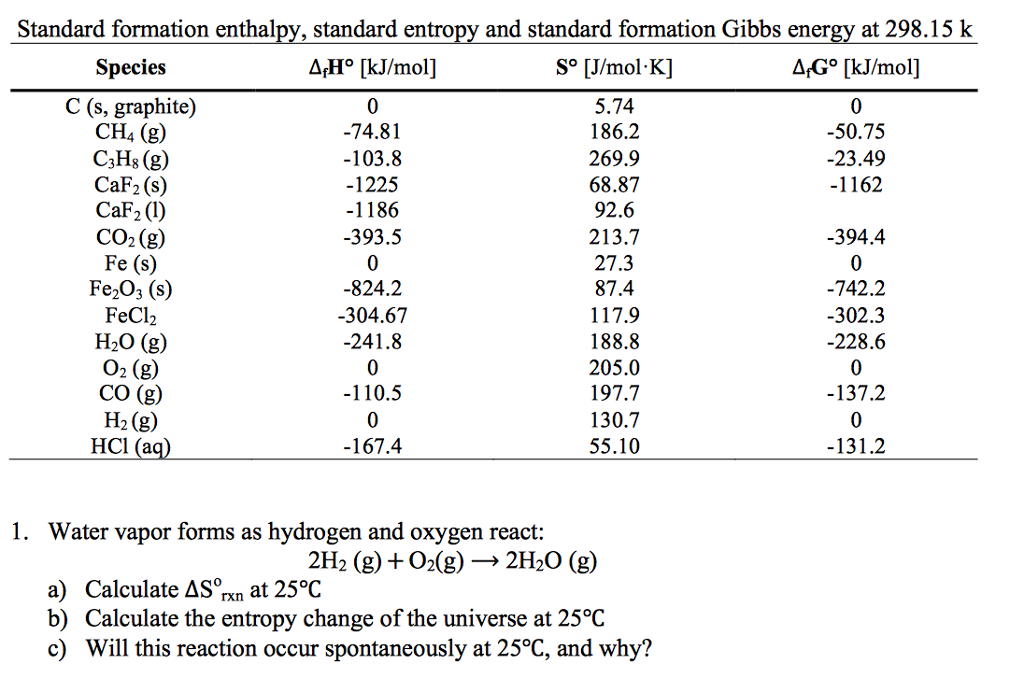
Delta S Sum Standard Molar Entropies of Products - Sum of Standard Molar Entropies of. It is apparent that entropies generally increase with molecular weight. 3) For the following reaction using the Thermodynamics table. Entropy, however, measures not energy itself, but its dispersal among the various quantum states available to accept it, and these exist even in pure elements. Scientists conventionally set the energies of formation of elements in their standard states to zero.


 0 kommentar(er)
0 kommentar(er)
